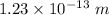Answer:
The wavelength is
Step-by-step explanation:
Given that,
Energy = 100 MeV
We need to calculate the De Broglie wavelength
Using formula of wavelength
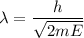
Where, h = Planck constant
m = mass of electron
E = Energy
Put the value into the formula
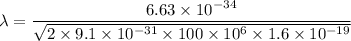
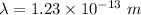
Hence, The wavelength is