Answer:
we will have 17.8 % of the original value
Step-by-step explanation:
As we know that by radioactive decay the total number of nuclei present at any instant of time is given as
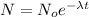
here we need to find the fraction of total number of nuclei present
so we will have
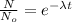
so we have
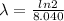
now we have
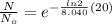
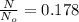
so we will have 17.8 % of the original value