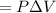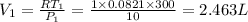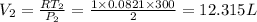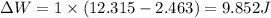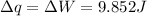Answer:
Step-by-step explanation:
Given
1 mole of perfect, monoatomic gas
initial Temperature
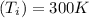
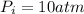
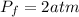
Work done in iso-thermal process
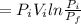
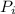 =initial pressure
=initial pressure
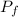 =Final Pressure
=Final Pressure
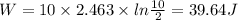
Since it is a iso-thermal process therefore q=w
Therefore q=39.64 J
(b)if the gas expands by the same amount again isotherm-ally and irreversibly
work done is