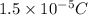Answer:
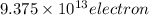 leave out with a charge of
leave out with a charge of
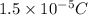
Step-by-step explanation:
We have given total charge

We know that charge on one electron =
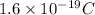
We have to find the total number of electron in total charge
So
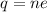 , here q is total charge, n is number of electron and e is charge on one electron
, here q is total charge, n is number of electron and e is charge on one electron
So
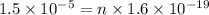
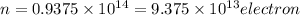
So
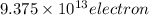 leave out with a charge of
leave out with a charge of