Answer:
The answer is B, HNO3 solution of 0.000015 M yields a pH of 4.8.
Step-by-step explanation:
The equation to find the pH of a solution is the following:
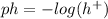
Since HNO3 is a strong acid, it almost 100% dissociates according to a 1:1 molar ratio, into hydrogen cations and nitrate anions, meaning the concentration of hydrogen ions is nearly the same as the concentration of the acid sollution.
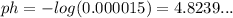
We can round that up to 4.8, meaning we have our answer, 0.000015 M.