Answer: The formula mass of caffeine is 97 amu and molar mass of caffeine is 194 g/mol
Step-by-step explanation:
Formula mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the empirical formula of a compound. It is expressed in amu.
Molar mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is expressed in g/mol.
Empirical formula is defined as the formula in which atoms in a compound are present in simplest whole number ratios.
The molecular formula of caffeine is
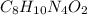
Dividing each number of atoms by '2', we will get the empirical formula of caffeine. The empirical formula of caffeine is
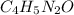
We know that:
Atomic mass of carbon = 12 amu
Atomic mass of hydrogen = 1 amu
Atomic mass of nitrogen = 14 amu
Atomic mass of oxygen = 16 amu
Formula mass of caffeine =
![(4* 12)+(5* 1)+(2* 14)+(1* 16)]=97amu](https://img.qammunity.org/2020/formulas/chemistry/college/gog78t5trl7u2efdln7a89k0lko3zukm0e.png)
Molar mass of caffeine =
![(8* 12)+(10* 1)+(4* 14)+(2* 16)]=194g/mol](https://img.qammunity.org/2020/formulas/chemistry/college/oc35n6p0ra62g7ea6ekgxt54qoskgnwhak.png)
Hence, the formula mass of caffeine is 97 amu and molar mass of caffeine is 194 g/mol