Answer:
24.525 g of sulfuric acid.
Step-by-step explanation:
Hello,
Normality (units of eq/L) is defined as:
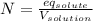
Since the sulfuric acid is the solute, and we already have the volume of the solution (500 mL) but we need it in liters (0.5 L, just divide into 1000), the equivalent grams of solute are given by:
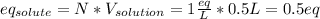
Now, since the sulfuric acid is diprotic (2 hydrogen atoms in its formula) 1 mole of sulfuric acid has 2 equivalent grams of sulfuric acid, so the mole-mass relationship is developed to find its required mass as follows:
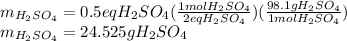
Best regards.