Answer:
ghdtgfgrdvreeeegrwwggegteefewqrwefrwerrftrtdsfgyuytfgererdf
Step-by-step explanation:
The given data is as follows.
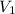 = 1.50 L,
= 1.50 L,
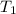 = 159 K,
= 159 K,
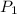 = 5.00 atm,
= 5.00 atm,
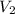 = 0.2 L,
= 0.2 L,
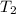 = ?,
= ?,
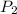 = 50.0 atm
= 50.0 atm
And, according to ideal gas equation,
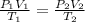
Hence, putting the given values into the above formula to calculate the value of final temperature as follows.
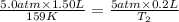
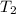 = 21.27 K
= 21.27 K
Thus, we can conclude that the final temperature is 21.27 K .