Answer : The correct option is, (b) R-COO-R'
Explanation :
Methyl ethanoate is also known as acetic acid methyl ester or methyl acetate. It is a carboxylate ester.
The formula of methyl ethanoate is,
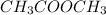
As we know that there are many functional groups which are:
(1) R-OH : It belongs to an alcoholic functional group in which the the -OH group is directly attached to the alkyl group of carbon.
(2) R-O-R' : It belongs to an ether functional group in which the the oxygen is directly attached to the two alkyl group of carbon.
(3) R-CO-R' : It belongs to ketone functional group in which the the -CO group is directly attached to the two alkyl group of carbon.
(4) R-CHO : It belongs to an aldehyde functional group in which the the -CHO group is directly attached to the alkyl group of carbon.
(5) R-COOH : It belongs to carboxylic acid functional group in which the the -COOH group is directly attached to the alkyl group of carbon.
(6) R-COO-R' : It belongs to ester functional group in which the the -COO group is directly attached to the two alkyl group of carbon.
From this we conclude that, the functional group found in methyl ethanoate is, ester.
Hence, the correct option is, (b) R-COO-R'