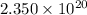Answer: The number of atoms of carbon present in given number of moles are
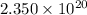
Step-by-step explanation:
We are given:
Number of moles of carbon =
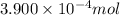
According to mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
So,
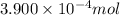 of carbon will contain =
of carbon will contain =
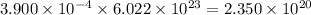 number of atoms.
number of atoms.
Hence, the number of atoms of carbon present in given number of moles are