Answer: Option (a) is the correct answer.
Step-by-step explanation:
A spontaneous reaction is defined as the reaction which occurs in the given set of conditions without any disturbance from any other source.
A spontaneous reaction leads to an increase in the entropy of the system. This means that degree of randomness increases in a spontaneous reaction.
For example,
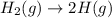
Here, 1 mole of hydrogen is giving 2 moles of hydrogen. This means that degree of randomness is increasing on the product side due to increase in number of moles.
Hence, there will also be increase in entropy.
Whereas in the reaction,
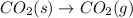 here number of moles remain the same. Hence, the reaction is not spontaneous.
here number of moles remain the same. Hence, the reaction is not spontaneous.
Thus, we can conclude that the reaction
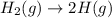 is spontaneous at STP.
is spontaneous at STP.