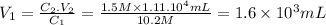Answer:
The required volume is 1.6 x 10³mL.
Step-by-step explanation:
When we want to prepare a dilute solution from a concentrated one, we can use the dilution rule to find out the required volume to dilute. This rule states:
C₁ . V₁ = C₂ . V₂
where,
C₁ and V₁ are the concentration and volume of the concentrated solution
C₂ and V₂ are the concentration and volume of the dilute solution
In this case, we want to find out V₁:
C₁ . V₁ = C₂ . V₂