Answer: The number of moles of gas present is 0.276 moles
Step-by-step explanation:
To calculate the number of moles of gas, we use the equation given by ideal gas:
PV = nRT
where,
P = Pressure of the gas = 725 mm Hg
V = Volume of the gas = 7.55 L
n = number of moles of gas = ?
R = Gas constant =
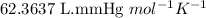
T = Temperature of the gas =
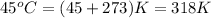
Putting values in above equation, we get:
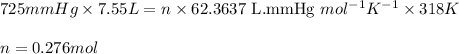
Hence, the number of moles of gas present is 0.276 moles