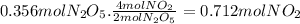Answer:
0.712 moles of NO₂ are formed.
Step-by-step explanation:
First, we need to write the balanced equation:
2 N₂O₅(g) ⇄ 4 NO₂(g) + O₂(g)
From the balanced equation, we can see the relationship between the moles of N₂O₅ and the moles of NO₂. Every 2 moles of N₂O₅ that react, 4 moles of NO₂ are formed. Let us apply this relationship to the information given by the problem (0.356 moles of N₂O₅):