Answer: The molarity of sodium sulfide and magnesium sulfide solution is 0.308M and 0.503 M respectively.
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
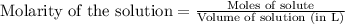 .....(1)
.....(1)
Or,
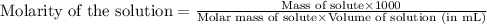 ......(2)
......(2)
Moles of sodium sulfide = 0.400 moles
Volume of solution = 1.30 L
Putting values in equation 1, we get:
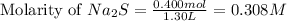
Hence, the molarity of sodium sulfide solution is 0.308 M
Mass of MgS = 23.9 g
Molar mass of MgS = 56.4 g/mol
Volume of solution = 843 mL
Putting values in equation 2, we get:
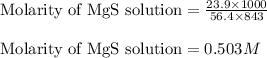
Hence, the molarity of magnesium sulfide solution is 0.503 M