Answer :
(1) pH = 1.27
(2) pH = 13.35
(3) The given solution is not a buffer.
Explanation :
(1) 53.1 mM HCl
Concentration of HCl =
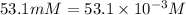
As HCl is a strong acid. So, it dissociates completely to give hydrogen ion and chloride ion.
So, Concentration of hydrogen ion=
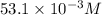
pH : It is defined as the negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
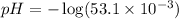
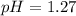
(2) 0.223 M KOH
Concentration of KOH = 0.223 M
As KOH is a strong base. So, it dissociates completely to give hydroxide ion and potassium ion.
So, Concentration of hydroxide ion= 0.223 M
Now we have to calculate the pOH.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
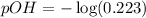
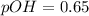
Now we have to calculate the pH.
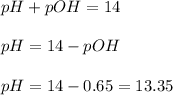
(3) 53.1 mM HCl + 0.223 M KOH
Buffer : It is defined as a solution that maintain the pH of the solution by adding the small amount of acid or a base.
It is not a buffer because HCl is a strong acid and KOH is a strong base. Both dissociates completely.
As we know that the pH of strong acid and strong base solution is always 7.
So, the given solution is not a buffer.