Step-by-step explanation:
Propanol is
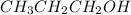 , whereas propane is
, whereas propane is
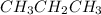 .
.
There is a presence of hydroxyl group in propanol which leads to the formation of hydrogen bonds with water and thus become soluble in water. Such bonding do not exists in propane.
Hydrogen bonding is a special type of the dipole-dipole interaction and it occurs between hydrogen atom that is bonded to highly electronegative atom which is either fluorine, oxygen or nitrogen atom.
Partially positive end of the hydrogen atom is attracted to partially negative end of these atoms which is present in another molecule. It is strong force of attraction between the molecules.