Step-by-step explanation:
The given data is as follows.
Initial concentration = 0.15 mg/ml,
Final concentration = 0.1 mg/ml, (as
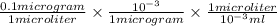 )
)
Final volume =
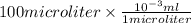 = 0.1 ml
= 0.1 ml
According to the dilution formula we get the following.
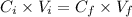
or,
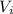 =
=
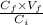
Putting the given values into the above formula we get the following.
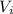 =
=
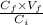
=
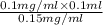
= 0.0667 ml
=
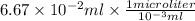
= 66.7 microliter
This means that volume of protein stock which is required is 66.7 ml. Hence, calculate the volume of water required as follows.
Volume of water required = Total volume - volume of protein stock
= (100 - 66.7) microliter
= 33.3 microliter
Thus, we can conclude that we need 33.3 microliter of water and 66.7 microliter of protein.