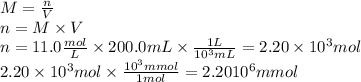Answer:
The chemist has added 2.20 x 10⁶ millimoles of silver perchlorate.
Step-by-step explanation:
Molarity is equal to the moles of solute per litre of solution.
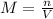
where,
n are the moles of solute
V is the volume of solution (expressed in litres)
From this expression, we can find out the moles of solute.