Step-by-step explanation:
The given data is as follows.
n = 2 mol, P = 1 atm, T = 300 K
Q = +34166 J, W= -1216 J (work done against surrounding)
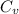 =
=
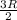
Relation between internal energy, work and heat is as follows.
Change in internal energy (
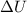 ) = Q + W
) = Q + W
= [34166 + (-1216)] J
= 32950 J
Also,
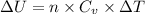
=
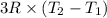
32950 J =
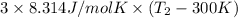
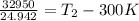
1321.06 K + 300 K =
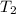
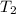 = 1621.06 K
= 1621.06 K
Thus, we can conclude that the final temperature of the gas is 1621.06 K.