Step-by-step explanation:
The given data is as follows.
P = 3 atm
=
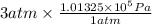
=
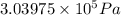
 = 9 L =
= 9 L =
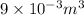 (as 1 L = 0.001
(as 1 L = 0.001
 ),
),
 = 15 L =
= 15 L =
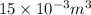
Heat energy = 800 J
As relation between work, pressure and change in volume is as follows.
W =
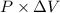
or, W =
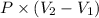
Therefore, putting the given values into the above formula as follows.
W =
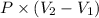
=
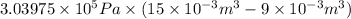
= 1823.85 Nm
or, = 1823.85 J
As internal energy of the gas
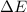 is as follows.
is as follows.
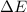 = Q - W
= Q - W
= 800 J - 1823.85 J
= -1023.85 J
Thus, we can conclude that the internal energy change of the given gas is -1023.85 J.