Answer:
6,666.66 micro liter of water and protein stock we will need to add to obtain the target concentration and volume.
Step-by-step explanation:
Concentration of given solution
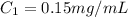
1 mL = 1000 μL , 1 mg = 1000 μg
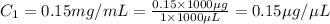
The volume of the given solution =
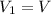
Concentration of required solution =
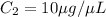
Volume of required solution =
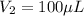
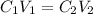
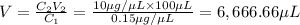
6,666.66 micro liter of water and protein stock we will need to add to obtain the target concentration and volume.