Answer:
We need 2.933 L of 0.15 mg /mL of protein solution.
Step-by-step explanation:
Concentration of given solution
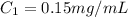
1 mg = 0.001 g , 1 mL = 0.001 L
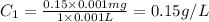
Molecular weight of protein = 22,000 Da =22,000 g/mol
Initial concentration in moles/liter:
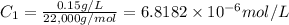
Initial concentration in micromoles/mL :
1 L = 1000 mL
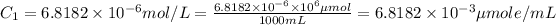
Initial concentration in micromoles/microLiter :
1 L = 1000,000 μL
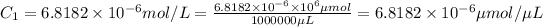
Moles of protein required = 20 μmoles
n(Moles)=C(concentration) × V(Volume of solution)
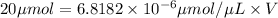
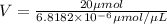
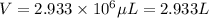
We need 2.933 L of 0.15 mg /mL of protein solution.