Answer:
Are produced 12,1 g of AlCl₃ and 5,33 g of Al₂O₃ are left over
Step-by-step explanation:
For the reaction:
Al₂O₃ (s) + 6 HCl (aq) → 2AlCl₃ (aq) + 3H₂0(l)
10,0g of Al₂O₃ are:
10,0g ₓ
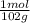 = 0,0980 moles
= 0,0980 moles
And 10,0g of HCl are:
10,0 gₓ
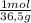 = 0,274 moles
= 0,274 moles
For a total reaction of 0,274 moles of HCl you need:
0,274×
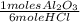 = 0,0457 moles of Al₂O₃
= 0,0457 moles of Al₂O₃
Thus, limiting reactant is HCl
The grams produced of AlCl₃ are:
0,274 moles HCl ×
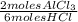 × 133
× 133
 = 12,1 g of AlCl₃
= 12,1 g of AlCl₃
The moles of Al₂O₃ that don't react are:
0,0980 moles - 0,0457 moles = 0,0523 moles
And its mass is:
0,0523 molesₓ
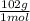 = 5,33 g of Al₂O₃
= 5,33 g of Al₂O₃
I hope it helps!