Answer:
1. The expression is:
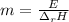
2. The computed mass is:
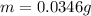
Step-by-step explanation:
Hello,
In this case, we know the so called enthalpy of reaction whose symbol and value is shown below:
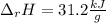
In addition, we know that the energy released by the involved reactant is:
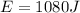
Therefore, the expression to compute the required mass, based on the given units is:
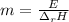
Finally, the computed mass turns out:
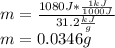
Best regards.