Answer:
The answer is 916.67 g
Step-by-step explanation:
48.0 wt% NaOH means that there are 48 g of NaOH in 100 g of solution. With this information and the molecular weight of NaOH (40 g/mol), we can calculate the number of mol there are in 100 g of this solution:
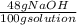 x
x
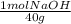 = 0.012 mol NaOH/100 g solution
= 0.012 mol NaOH/100 g solution
Finally, we need 0.11 mol in 1 liter of solution to obtain a 0.11 M NaOH solution.
0.012 mol NaOH ------------ 100 g solution
0.11 mol NaOH------------------------- X
X= 0.11 mol NaOH x 100 g/ 0.012 mol NaOH= 916.67 g
We have to weigh 916.67 g of 48.0%wt NaOH and dilute it in a final volume of 1 L of water to obtain a 0.11 M NaOH solution.