Answer:
You have to weigh 0.079 g of Na₂S₂0₃ and dissolve it in water at a final volume of 250 ml.
Step-by-step explanation:
A 0.00200 M Na₂S₂O₃ solution has 0.002 mol of Na₂S₂O₃ per liter of solution. As we know that 1 L = 1000 ml, and that the molecular weight of Na₂S₂O₃ IS 158 g/mol, we can calculate the mass of Na₂S₂O₃ to weigh as follows:
mass=
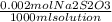 x
x
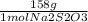 x 250 ml solution
x 250 ml solution
mass= 0.079 g
To prepare the solution, we have to weigh in a beaker with 0.079 g of Na₂S₂O₃ by employing an analytical balance. Then, we have to dissolve the mass in a volume of aproximately 230 ml water. For this, we measure the 230 ml of water in a graduated cylinder, we add the volume to the beaker with the mass and we agitate until total disolution. Finally, we tranfer the total amount of the solution in the glass to a volumetric flask with a capacity of 250 ml. We add water until we reach the capacity, and then we homogenize the solution.