Answer:
2.32 m
Step-by-step explanation:
So, according to definition of mole fraction:
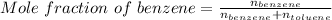
Mole fraction = 0.176
Applying values as:
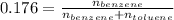
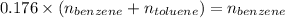
So,

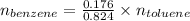
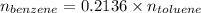
Also, Molar mass of toluene = 92.14 g/mol
Thus,
The formula for the calculation of moles is shown below:

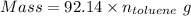
Also, 1 g = 0.001 kg
So,
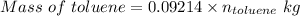
Molality is defined as the moles of the solute present in 1 kg of the solvent.
It is represented by 'm'.
Thus,
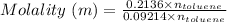
Molality of benzene = 2.32 m