Answer:
The solubility of methylacetylene is 0,11 g L⁻¹
Step-by-step explanation:
Henry's law is a gas law that states that the amount of dissolved gas in a liquid is proportional to its partial pressure above the liquid.
The formula is:
C = kH P
Where C is solubility of the gas (In mol/L)
kH is Henry constant (9,23x10⁻² mol L⁻¹ atm⁻¹)
An P is partial pressure (0,301 atm)
Solving, C = 2,78x10⁻³ mol L⁻¹. In grams per liter:
2,78x10⁻³ mol L⁻¹ₓ
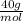 = 0,11 g L⁻¹
= 0,11 g L⁻¹
I hope it helps!