Answer: 72.41% and 26.90% respectively.
Step-by-step explanation:
At 60°C, you can dissolve 46.4g of acetanilide in 100mL of ethanol. If you lower the temperature, at 0°C, you can dissolve just 12.8g, which means (46.4g-12.8g)=33.6g of acetanilide must have precipitated from the solution.
We can calculate recovery as:
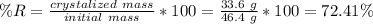
So the answer to the first question is 72.41%.
For the second part just use the same formula, the mass of the precipitate is the final mass minus the initial mass, (171mg-125mg)=46mg.
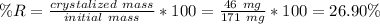
So the answer to the second question is 26.90%.