Answer: The metal having molar mass equal to 26.95 g/mol is Aluminium
Step-by-step explanation:
- To calculate the number of moles for given molarity, we use the equation:
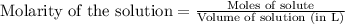 .....(1)
.....(1)
Molarity of NaOH solution = 0.5000 M
Volume of solution = 0.03340 L
Putting values in equation 1, we get:
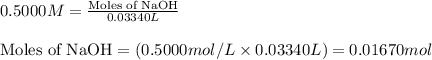
- The chemical equation for the reaction of NaOH and sulfuric acid follows:

By Stoichiometry of the reaction:
2 moles of NaOH reacts with 1 mole of sulfuric acid
So, 0.01670 moles of NaOH will react with =
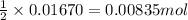 of sulfuric acid
of sulfuric acid
Excess moles of sulfuric acid = 0.00835 moles
- Calculating the moles of sulfuric acid by using equation 1, we get:
Molarity of sulfuric acid solution = 0.5000 M
Volume of solution = 127.9 mL = 0.1279 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
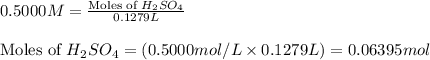
Number of moles of sulfuric acid reacted = 0.06395 - 0.00835 = 0.0556 moles
- The chemical equation for the reaction of metal (forming
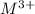 ion) and sulfuric acid follows:
ion) and sulfuric acid follows:
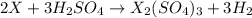
By Stoichiometry of the reaction:
3 moles of sulfuric acid reacts with 2 moles of metal
So, 0.0556 moles of sulfuric acid will react with =
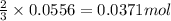 of metal
of metal
- To calculate the molar mass of metal for given number of moles, we use the equation:

Mass of metal = 1.00 g
Moles of metal = 0.0371 moles
Putting values in above equation, we get:
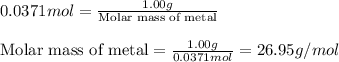
Hence, the metal having molar mass equal to 26.95 g/mol is Aluminium