Answer:
The grams of chlorophyll you need are 11,1 g
Step-by-step explanation:
Osmotic pressure is the minimum pressure that you needs to be apply to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. The formula of osmotic pressure is:
π = M×R×T
Where:
M is molar concentration of dissolved species (units of mol/L).
R is ideal gas constant (0.08206 L atm mol⁻¹ K⁻¹, ).
T is the temperature on the Kelvin scale.
In the problem you have:
1,19 atm = M×0,08206 L atm mol⁻¹ K⁻¹×298K
M = 0,048 M
Thus, the grams of chlorophyll you need are:
0,048
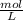 × 0,258 L ×
× 0,258 L ×
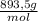 = 11,1 g
= 11,1 g
I hope it helps!