Answer:
895.8g/mol
Step-by-step explanation:
Hello, here the solution:
Consider the formula for the boiling-point elevation:
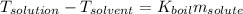
We solve for the molality of the solute:
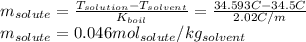
By knowing the mass of the diethyl ether (271.8 g =0.2718kg) and that 11.20g of the solute were dissolved, the molecular mass is given by:
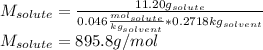
Best regards.