Answer: The net chemical reaction:
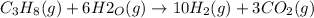
Step-by-step explanation:
Step 1 : Propane reacts with water to form carbon monoxide and hydrogen gas
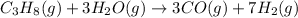 ...[1]
...[1]
Step 2 : carbon monoxide formed in above step reacts with water tio form hydrogen gas and carbon dioxide.
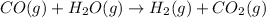 ...[2]
...[2]
On Adding [1] and 3 × [2] we get overall balanced chemical reaction for the production of hydrogen from propane and water:
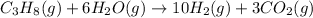
1 mole of propane reacts with 6 moles of water vapor to gve 10 moles of hydrogen gas and 3 moles of carbon dioxide.