Answer:
0.7246 M
Step-by-step explanation:
Considering:

Or,
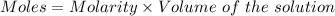
Given :
For
 :
:
Molarity = 2.086 M
Volume = 188.9 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 188.9×10⁻³ L
Thus, moles of
 :
:
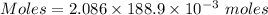
Moles of
 = 0.39405 moles
= 0.39405 moles
For NaOH :
Molarity = 0.4607 M
Volume = 269.3 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 269.3×10⁻³ L
Thus, moles of NaOH :
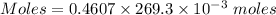
Moles of NaOH = 0.1241 moles
According to the given reaction:
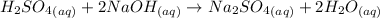
1 moles of
 react with 2 moles of NaOH to form 1 mole of sodium sulfate.
react with 2 moles of NaOH to form 1 mole of sodium sulfate.
Thus,
2 moles of NaOH react with 1 mole of

1 mole of NaOH react with 1/2 mole of

0.1241 moles of NaOH react with (1/2)×0.1241 mole of

Moles of
 that got reacted = 0.06205 moles
that got reacted = 0.06205 moles
Unreacted moles = Total moles - Moles that got reacted = 0.39405 - 0.06205 moles = 0.332 moles
Total volume = 188.9×10⁻³ L + 269.3×10⁻³ L = 458.2×10⁻³ L
Concentration of
 :
:

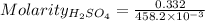
Concentration of
 = 0.7246 M
= 0.7246 M