Answer:
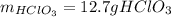
Step-by-step explanation:
Hello,
Considering the reaction:

The molar masses of chlorine and chloric acid are:
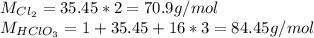
Now, we develop the stoichiometric relationship to find the mass of chloric acid, considering the molar ratio 3:1 between chlorine and chloric acid, as follows:
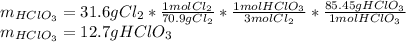
Best regards.