Answer:
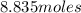
Step-by-step explanation:
We have got a balanced reaction equation.What information can we obtain from it?
The numbers on the left from each substance indicate us what amount of substance react and what amount of substance is formed.
In the equation :
2N2H4(g) + N2O4(g) → 3N2(g) + 4H2O(g)
2 moles of N2H4(g) + 1 mol of N2O4(g) is combined to form 3 moles of N2(g) + 4 moles of H2O(g)
We have got excess from N2O4 so N204 does not limit us to form N2
2 moles of N2H4 → 3 moles of N2
5.89 moles of N2H4 → X =
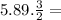
X = 8.835 moles N2