Answer:
1,706 M of HCl
Step-by-step explanation:
In the reaction:
2 HCl + Na₂CO₃ → 2 NaCl + CO₂ + H₂O
The moles of HCl are:
178,5mL≡0,1785 L×2,461 M =0,4393 moles of HCl
The moles of Na₂CO₃ are:
7,140 g ÷ 105.9888 g/mol = 0,06737 moles of Na₂CO₃
In a total reaction of 0,06737 moles of Na₂CO₃ you need:
0,06737 moles of Na₂CO₃ ×
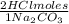 = 0,1347 moles of HCl
= 0,1347 moles of HCl
Thus, the restant moles of HCl are:
0,4393 moles - 0,1347 moles = 0,3046 moles of HCl
Thus, molar concentration of HCl is:
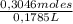 = 1,706 M of HCl
= 1,706 M of HCl
I hope it helps!