Answer : The answer will be 20.68
Explanation :
Significant figures : The figures in a number which express the value -the magnitude of a quantity to a specific degree of accuracy is known as significant digits.
The rule apply for the multiplication and division is :
The least number of significant figures in any number of the problem determines the number of significant figures in the answer.
The rule apply for the addition and subtraction is :
The least precise number present after the decimal point determines the number of significant figures in the answer.
The given expression is:
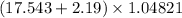
In the given expression, 17.543 has 5 significant figures and 2.19 has 3 significant figures. From this we conclude that least precise number present after the decimal point is 2. So, the answer will be:
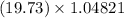
In the given expression, 19.73 has 4 significant figures and 1.04821 has 6 significant figures. From this we conclude that 4 is the least significant figures in this problem. So, the answer should be in 4 significant figures.
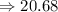
Thus, the answer will be 20.68