Answer:
52.2538 psia
Step-by-step explanation:
The absolute pressure at depth of 27 inches can be calculated by:
Pressure = Local pressure + Gauge pressure
Also,
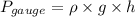
Where,
 is the density of glycerin (
is the density of glycerin (
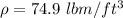 )
)
g is the gravitational acceleration = 32.1741 ft/s²
h = 27 in
Also, 1 in = 1/12 ft
So,
h = 27 / 12 ft = 2.25 ft
So,
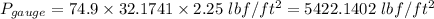
Also,
1 ft = 12 inch
1 ft² = 144 in²
So,
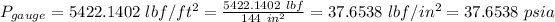
Local pressure = 14.6 psia
So,
Absolute pressure = 14.6 psia + 37.6538 psia=52.2538 psia