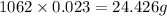Answer:
Mass of the substance is 24.426 g.
Step-by-step explanation:
Given that the density is
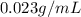
The volume of substance is
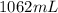 .
.
density of a substance is the mass of the substance per unit volume.
density=
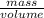
In this question the mass of the substance is
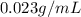
Therefore the mass of the substance in
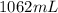 of the substance is given by
of the substance is given by
mass =
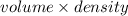
mass =