Answer:
so specific weight is 009 lb-f/ft³
Step-by-step explanation:
given data
temperature = 100°F = 559.67°R
absolute pressure = 60 psi = 8640 lb/ft²
gas constant for the air R = 1716 ft⋅lb/[slug⋅R]
to find out
specific weight of air
solution
we know specific weight is express as
specific weight =
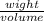
specific weight =
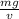 ...........1
...........1
m is mass and v is volume and g is acceleration due to gravity = 32.174 ft/s²
and by ideal gas equation
Pv = nRT
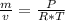
so specific weight
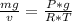
specific weight =
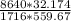
specific weight = 0.2894472 slug/ft²-s²
specific weight = 9.312677 lbm/ft²-s²
so specific weight is 009 lb-f/ft³