Answer:
9.7g / cm^3
Step-by-step explanation:
To calculate a conbined density we must find the ratio between the sum of the masses and the sum of the volumes remembering that the equation to find the density is α=m/v, taking into account the above the following equation is inferred.
αc=copper density
αs=silver density
Vs=volume of silver
Vc=volume of copper
α= density of alloy
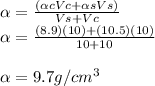
the density of the alloy is 9.7g / cm^3