Answer with Explanation:
The capillary rise in 2 parallel plates immersed in a liquid is given by the formula
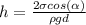
where
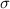 is the surface tension of the liquid
is the surface tension of the liquid
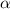 is the contact angle of the liquid
is the contact angle of the liquid
 is density of liquid
is density of liquid
'g' is acceleratioj due to gravity
'd' is seperation between thje plates
Part a) When the liquid is water:
For water and glass we have
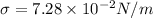
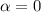
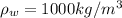
Applying the values we get
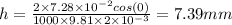
Part b) When the liquid is mercury:
For mercury and glass we have

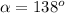

Applying the values we get
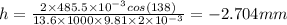
The negative sign indicates that there is depression in mercury in the tube.