Answer:
gauge pressure is 133 kPa
Step-by-step explanation:
given data
initial temperature T1 = 27°C = 300 K
gauge pressure = 300 kPa = 300 × 10³ Pa
atmospheric pressure = 1 atm
final temperature T2 = 77°C = 350 K
to find out
final pressure
solution
we know that gauge pressure is = absolute pressure - atmospheric pressure so
P (gauge ) = 300 × 10³ Pa - 1 ×
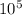 Pa
Pa
P (gauge ) = 2 ×
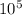 Pa
Pa
so from idea gas equation
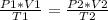 ................1
................1
so
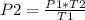
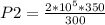
P2 = 2.33 ×
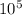 Pa
Pa
so gauge pressure = absolute pressure - atmospheric pressure
gauge pressure = 2.33 ×
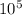 - 1.0 ×
- 1.0 ×
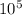
gauge pressure = 1.33 ×
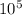 Pa
Pa
so gauge pressure is 133 kPa