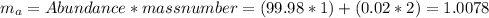Answer:
The answer is B
Step-by-step explanation:
The atomic mass (
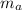 ) is the average mass of all the isotopes an element has, therefore it is unique, an in this case the element will be Halfnium, as the number is 178.
) is the average mass of all the isotopes an element has, therefore it is unique, an in this case the element will be Halfnium, as the number is 178.
The atomic mass however, does not relate to the number of protons and neutrons directly, given that the atomic mass takes all the isotopes into account.
The mass number (A) on the other hand, does consider the number of protons and neutrons. The definition of mass number is A=N° neutrons+N° protons.
Let us consider the Hydrogen as an example. Hydrogen has three isotopes:
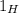 which abundance is 99.98%
which abundance is 99.98%
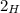 which abundance is 0.02%
which abundance is 0.02%
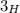 (traces)
(traces)
In this case, the mass number for each one of the isotopes, will be 1, 2 and 3 respectively. However, the atomic mass will be the average mass of the three isotopes, therefore: