Answer:
9 moles of particles are present in 1 mole of ethyl alcohol.
Step-by-step explanation:
1. Ethanol is ethyl alcohol with the chemical formula
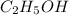
2. Molecular formula is given by
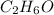
3.
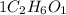
4. 1 represents 1 mole of ethyl alcohol
5. Subscripts represents the number of moles of each element.
6. 2 moles of carbon atoms, 6 moles of Hydrogen atoms and 1 mole of oxygen atoms are present in 1 mole of ethyl alcohol.
7. So, totally 9 moles of particles are present in 1 mole of ethyl alcohol
8. 1 mole contains
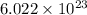 particles or atoms or molecules or ions
particles or atoms or molecules or ions
9. For example 1 mole of
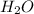 contains
contains
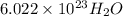 molecules (contains 2 moles of H and 1 mole of O atoms )
molecules (contains 2 moles of H and 1 mole of O atoms )
10. 1 mole of Na contains
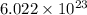 Na atoms
Na atoms
11. 1 mole of
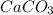 contains
contains
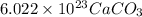 molecules (1 mole of Ca, 1mole of C and 3 moles of O atoms )
molecules (1 mole of Ca, 1mole of C and 3 moles of O atoms )
12. 1 mole of
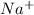 contains
contains
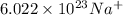 ions
ions