Answer:
R_2 = 7.647 m
Step-by-step explanation:
HI!
Let us consider that the aballon is filled with a gas that follows the ideal gas equation. Since the amount (number of moles) of the gas is constant we should have:
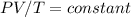
Therefore, we can have the following relationship between two differnet states given their Volume, Pressure and Tempreature:
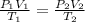
Since the volume of a sphere is
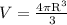
The relathionship will be:
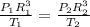
Solving for R_2:
![R_2 = R_1 \sqrt[3]{(P_1 T_2)/(P_2 T_1)}](https://img.qammunity.org/2020/formulas/physics/high-school/zzzl13hmb3kz070xrmsle2jhnl009j0q8x.png)
Where the index 2 is the state at the lift-off and the index 1 denotes the state at its maximum radius. Replacing all the values given we find that:
R_2 = 7.647 m