Answer : The final chamber pressure is 0.746 atm.
Step-by-step explanation:
First we have to calculate the moles of
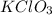 .
.
Molar mass of
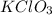 = 122.5 g/mole
= 122.5 g/mole
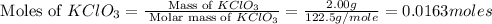
Now we have to calculate the moles of
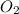 .
.
The balanced chemical reaction will be:
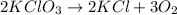
From the balanced reaction we conclude that,
As, 2 moles of
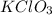 react to give 3 moles of
react to give 3 moles of
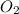
So, 0.0163 moles of
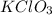 react to give
react to give
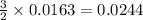 moles of
moles of
Now we have to calculate the pressure of gas.
Using ideal gas equation:

where,
P = pressure of gas = ?
V = volume of gas = 0.800 L
T = temperature of gas =
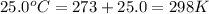
R = gas constant = 0.0821 L.atm/mole.K
n = number of moles of gas = 0.0244 mole
Now put all the given values in the ideal gas equation, we get:
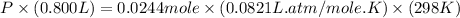
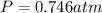
Therefore, the final chamber pressure is 0.746 atm.