Answer:
The answer to your question is below:
Explanation :
The combined gas law as it name says it combines three gas laws: Boyle's law, Charles' law and Gay Lussac Law. It states that at initial conditions of pressure, volume and temperature, it there is a change in one of this variables
the equilibrium will be equivalent to a second equilibria of pressure, volume and temperature.
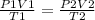
"1" indicates initial conditions
"2" indicates final conditions